If you have been reading the newsletter and blogs on this page you know we are now in the Golden Age of Nutrition. Scientists have finally come to realize what the Ancients knew very well: AS ABOVE SO BELOW.
Their understanding of the Sun’s meaning in our lives and that of the planets is now clear to Physicists: the Energy and Information we get from the Sun sustains ALL THINGS ON EARTH. Energy and Information from the Sun fuel our societies AND our body’s 50 trillion cells. Thermodynamics laws apply in our engines and cells equally. Indeed, “There is nothing new under the Sun.”

The ancients symbolized Energy and Information in many ways. The best known symbols are The Cross and The Caduceus. The former is best known to the religiously inclined while the latter is a symbol of the Medical profession. The Caduceus depicts two snakes (Energy and Information) coiling around a central staff for BALANCE. Balance is also known as HOMEOSTASIS, another word for HEALTH. In other words, a balance of the forces of Nature, Energy and Information, maintains health.

Think of food as Energy and Information. All food is rooted in Photosynthesis, or the process whereby plants soak up Energy and Information. When we eat good food we are maximizing the fueling of our 50 trillion cells AND how our genes manifest themselves. This process also replaces the 10 billion cells we lose daily. Just like Nature is a constant process of Creation-Destruction, our body is “recycling” itself as we speak. The Ancients symbolized this by the Auroboros in Mesopotamia, and the Dance of Shiva in India. In medicine we call these processed Metabolism-Catabolism; they are also intimately linked to the laws of Thermodynamics.

All the above comes to a sharp focus in the gut, which is where we digest the food we eat AND determine what substance is allowed to become part of ourselves. These are some of the basic functions of the Immuno-detoxification system represented in great part by the gut bacteria that keeps us alive.

Once we understand these principles we have no choice but to address health concerns from a nutritional point of view first. Drugs are then seen as emergency treatments designed to maintain life in dire situations, and not as a cure for the consequences of poor diets, toxic environments and stressful relationships.

The above concepts are now found in the best of medical and scientific journals on a weekly basis. Of course, they are written in modern language, but, a careful study of their contents will corroborate the simple and ancient truths noted above. For those who care to read some of them, here they are:
“Nutrient and immune sensing are obligate pathways in metabolism, immunity, and disease,” September 2015 The FASEB Journal vol. 29 no. 9 3612-3625
“The growth and survival of multicellular organisms depend upon their abilities to acquire and metabolize nutrients, efficiently store and harness energy, and sense and fight infection. Systems for sensing and using nutrients have consequently coevolved alongside systems for sensing and responding to danger signals, including pathogens, and share many of the same cell signaling proteins and networks. Diets rich in carbohydrates and fats can overload these systems, leading to obesity, metabolic dysfunction, impaired immunity, and cardiovascular disease. Excessive nutrient intake promotes adiposity, typically altering adipocyte function and immune cell distribution, both of which trigger metabolic dysfunction. Here, we discuss novel mechanistic links between metabolism and immunity that underlie metabolic dysfunction in obesity. We aim to stimulate debate about how the endocrine and immune systems are connected through autocrine, paracrine, and neuroendocrine signaling in sophisticated networks that are only now beginning to be resolved. Understanding the expression and action of signaling proteins, together with modulating their receptors or pattern recognition using agonists or antagonists, will enable rational intervention in immunometabolism that may lead to novel treatments for obesity and metabolic dysfunction.”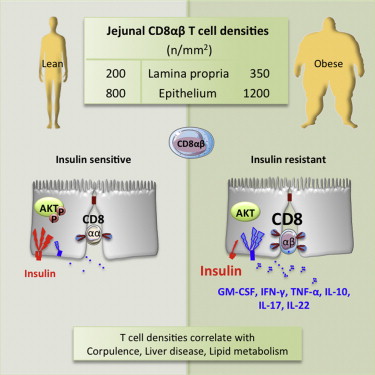
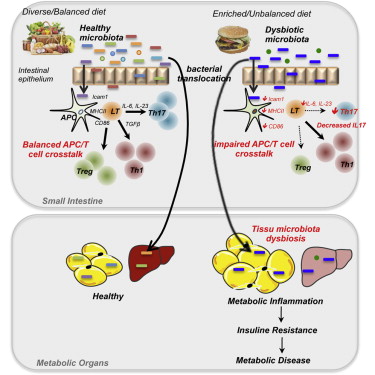
“Metabolic Flexibility and Dysfunction in Cardiovascular Cells,” J. Arterioscler Thromb Vasc Biol. 2015;35:e37
“Cardiovascular cells that contribute directly to atherosclerosis and cardiac dysfunction are known to exhibit metabolic flexibility, characterized by the ability to switch from generating ATP primarily through oxidative phosphorylation to using glycolysis as the predominate energy source, and to shift from one fuel source to another. This flexibility occurs in endothelial cells (ECs), myeloid cells, and cardiomyocytes during normal development and physiology, and is thought to have evolved to protect cells with heightened energy demand from the increased oxidative stress that can be a result of oxidative phosphorylation,1 to shunt glucose to side branches of glycolysis,2 to provide energy more rapidly,1 or to use the most abundant fuel available.3 With the growing problem of systemic nutrient overload and associated insulin resistance, type 2 diabetes mellitus, and nonalcoholic fatty liver disease, metabolic flexibility and dysfunction in cells involved in cardiovascular disease have received increased attention as possible contributors to systemic inflammation and cardiovascular risk associated with these states. Systemic insulin resistance is thought to be due primarily to nutrient overload in skeletal muscle and liver as a consequence of an inability of adipose tissue to store excess nutrients in the form of triacylglycerol-rich lipid droplets, and a subsequent increase in detrimental lipid species in liver and skeletal muscle, which are inadequately equipped to store large amounts of lipid. Accumulation of noxious lipids leads to dysfunction in liver and skeletal muscle cells characterized by insulin resistance, increased activation of the unfolded protein response, and increased production of inflammatory [signals].”
“Longitudinal Associations Between Metabolic Syndrome Components and Telomere Shortening,” Journal Clinical Endocrinology Metabolism 2015;100(8), pp. 3050
“Metabolic dysregulations are associated with shorter telomeres over two time points. In particular, increasing abdominal adiposity is accompanied by accelerated telomere attrition. Future studies should elucidate underlying mechanisms of this bidirectional relationship and investigate whether targeting obesity may reduce telomere attrition to prevent further deterioration toward cardiovascular and aging-related complications.”


